RJ GAUDET & ASSOCIATES L.L.C.
"Let us realize the arc of the moral universe is long but it bends toward justice."
Dr. Martin Luther King, Jr.
Archive for February, 2015
No access to justice in the US for Dutch victims of vaginal mesh products
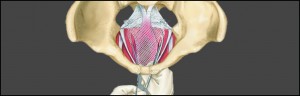
Recently, a Dutch TV show for consumers, Radar, featured a report on how easy it is for manufacturers of medical devices, such as transvaginal mesh products, to have their product approved for the European market. Back in 2012 this TV program broadcasted a show informing Dutch consumers about faulty transvaginal surgical mesh slings that were used to treat pelvic organ prolapse and stress and urinary incontinence.
In the United States, more than 40,000 patients individually brought cases against the manufacturers of these same defective medical devices. These cases from all over the US were assigned to one court, the United States District Court for the Southern District of West Virginia. By aggregating the cases through a special federal legal procedure called Multidistrict Litigation (MDL), the process of handling these complex cases has been speeded up.
 Of all these cases, at least twenty were filed by injured individuals from New Zealand. The defendants, manufacturers Ethicon, Inc. and Johnson & Johnson, Inc., moved to dismiss their claims based on the forum non conveniens doctrine. Forum non conveniens is a doctrine that permits a district court to dismiss (or transfer) a case if the current forum, the US, is inconvenient. Defendants have to show that there is an adequate alternative forum, meaning that forum (here: New Zealand), is (1) available, (2) adequate, and (3) more convenient in light of the public and private interests involved. [1]
Of all these cases, at least twenty were filed by injured individuals from New Zealand. The defendants, manufacturers Ethicon, Inc. and Johnson & Johnson, Inc., moved to dismiss their claims based on the forum non conveniens doctrine. Forum non conveniens is a doctrine that permits a district court to dismiss (or transfer) a case if the current forum, the US, is inconvenient. Defendants have to show that there is an adequate alternative forum, meaning that forum (here: New Zealand), is (1) available, (2) adequate, and (3) more convenient in light of the public and private interests involved. [1]
In the case of the New Zealanders, Judge Goodwin decided the motion to dismiss on February 18, 2014 (Solvander v. American Medical Systems, Inc., 2:13-cv-19418, MDL No. 2325, Doc. 13). The court granted the motion to dismiss, after deciding New Zealand fulfills all the requirements of an adequate alternative forum. Technically, the motion to dismiss was filed only by defendants Ethicon, Inc. and Johnson & Johnson, Inc. American Medical Systems (AMS) did not file a similar motion. However, most likely, the same holding would apply to the claims against AMS because the court’s February 18 decision was filed on the AMS docket and the same reasoning would apply.
After analyzing Judge Goodwin’s decision to dismiss the cases of the New Zealanders, RJ Gaudet & Associates LLC believes the court would similarly dismiss cases brought by injured Dutch victims against manufacturers of faulty medical devices in the US, at least as long as their circumstances are similar to the circumstances of those of the New Zealanders, e.g. they were treated outside the US.
As a summary, the US legal system most likely does not allow Dutch victims of faulty vaginal mesh products to claim damages in the US, as any case that would be filed in the US could be dismissed on request of defendants by the judge on the grounds of forum non conveniens. Dutch victims will have to pursue access to justice in the Netherlands, instead.
 As for the thousands of existing claims, on February 5, 2015, the court in West Virginia will hold a status conference. The court has required defendant client representatives “with full authority to make all decisions related to MDL 2325” against AMS to attend. In the US, RJ Gaudet & Associates LLC represents clients against AMS over defective transvaginal mesh products, e.g. the Monarc Hammock.
As for the thousands of existing claims, on February 5, 2015, the court in West Virginia will hold a status conference. The court has required defendant client representatives “with full authority to make all decisions related to MDL 2325” against AMS to attend. In the US, RJ Gaudet & Associates LLC represents clients against AMS over defective transvaginal mesh products, e.g. the Monarc Hammock.


